
Responsible for the day to day management of specific processes and personnel related to the BioFire Diagnostics (BFDX) Quality Assurance Systems. The data presented on this page does not represent the view of BioFire Diagnostics and its employees or that of Zippia.īioFire Diagnostics may also be known as or be related to BioFire Diagnostics, BioFire Diagnostics LLC, BioFire Diagnostics, LLC., Biofire Diagnostics, LLC and Biofire Diagnostics, LLC. In North America we have more than 5,000 team members across 11 sites or subsidiaries, including Salt Lake City-based BioFire Diagnostics and one subsidiary in Montreal, Canada. Biofire Diagnostics LLC, based in Salt Lake City, sets the standard for molecular diagnostics through its pioneering advances in syndromic infectious. None of the information on this page has been provided or approved by BioFire Diagnostics. While we have made attempts to ensure that the information displayed are correct, Zippia is not responsible for any errors or omissions or for the results obtained from the use of this information. Salary information comes from 44 data points collected directly from. Average Biofire Diagnostics hourly pay ranges from approximately 15.50 per hour for Material Handler to 23.48 per hour for Technician. Sources of data may include, but are not limited to, the BLS, company filings, estimates based on those filings, H1B filings, and other public and private datasets. The average Biofire Diagnostics salary ranges from approximately 30,435 per year for Material Handler to 86,621 per year for Engineer. The data on this page is also based on data sources collected from public and open data sources on the Internet and other locations, as well as proprietary data we licensed from other companies. The employee data is based on information from people who have self-reported their past or current employments at BioFire Diagnostics. It is meant for close-to-patient testing of cerebrospinal fluid specimens that are collected by a lumbar. The data presented on this page does not represent the view of BioFire Diagnostics and its employees or that of Zippia.īioFire Diagnostics may also be known as or be related to BioFire Diagnostics, BioFire Diagnostics LLC, BioFire Diagnostics, LLC., Biofire Diagnostics, LLC and Biofire Diagnostics, LLC.Zippia gives an in-depth look into the details of BioFire Diagnostics, including salaries, political affiliations, employee data, and more, in order to inform job seekers about BioFire Diagnostics. The BioFire FilmArray Meningitis/Encephalitis Panel was the first Food and Drug Administration-cleared multiplex polymerase chain reaction for the evaluation of cerebrospinal fluid samples, able to identify 14 organisms in a single test reaction. The FilmArray ME Panel from BioMérieux subsidiary BioFire Diagnostics is a one-hour, sample-to-answer test for 14 different pathogens associated with infectious meningitis or encephalitis, including common bacteria, viruses, and yeasts.
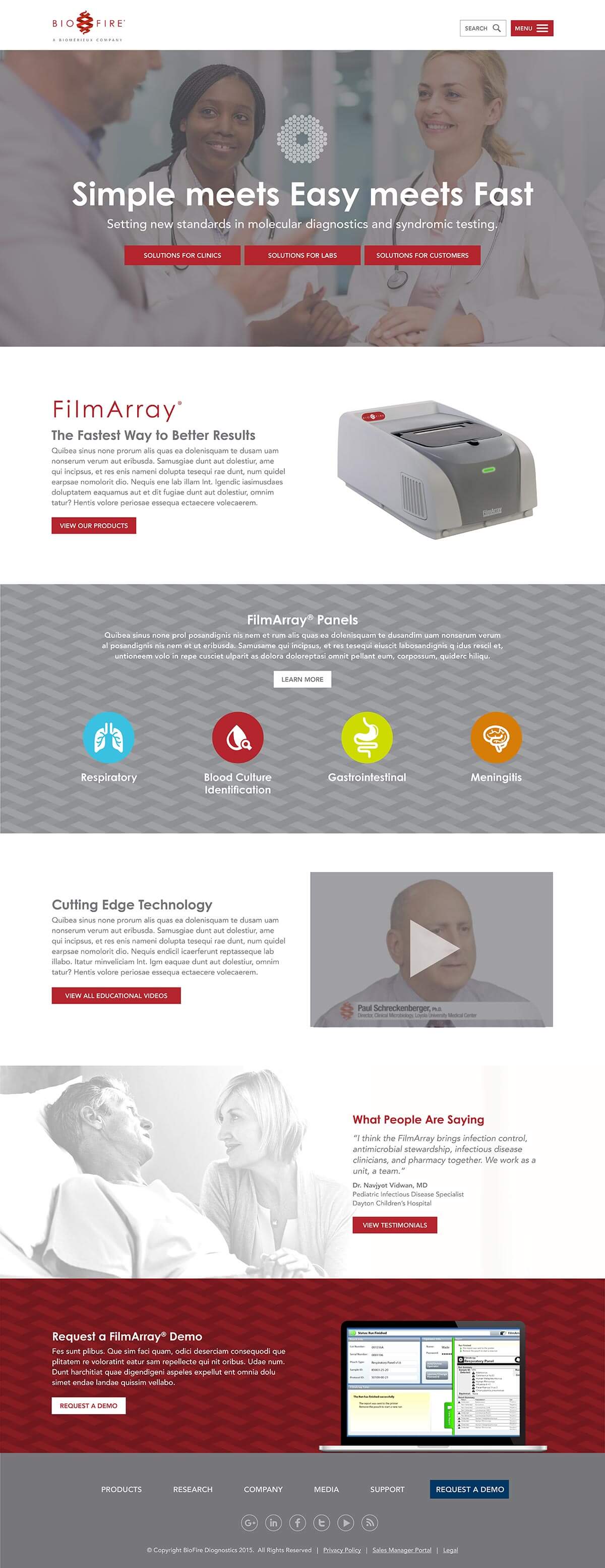
Sources of data may include, but are not limited to, the BLS, company filings, estimates based on those filings, H1B filings, and other public and private datasets. The employee data is based on information from people who have self-reported their past or current employments at BioFire Diagnostics. BioFire Diagnostics, headquartered in Salt Lake City, Utah, and established in 1990, is a clinical molecular diagnostic solutions provider specializing in. authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. The FilmArray meningitis/encephalitis (ME) panel (BioFire Diagnostics, LLC, Salt Lake City, UT, USA) was approved by the Food and Drug Administration (FDA).

Zippia gives an in-depth look into the details of BioFire Diagnostics, including salaries, political affiliations, employee data, and more, in order to inform job seekers about BioFire Diagnostics.


 0 kommentar(er)
0 kommentar(er)
